¶ ZIC-pHILIC METHOD DEVELOPMENT
Your content here
- 0.19 mL/min -> ~1.5 min -> 20 min
- 0.17 mL/min -> ~1.5 min -> 22 min
- Attempting to improve Chromatography via increased column temperature and using previously optomized flow rates.
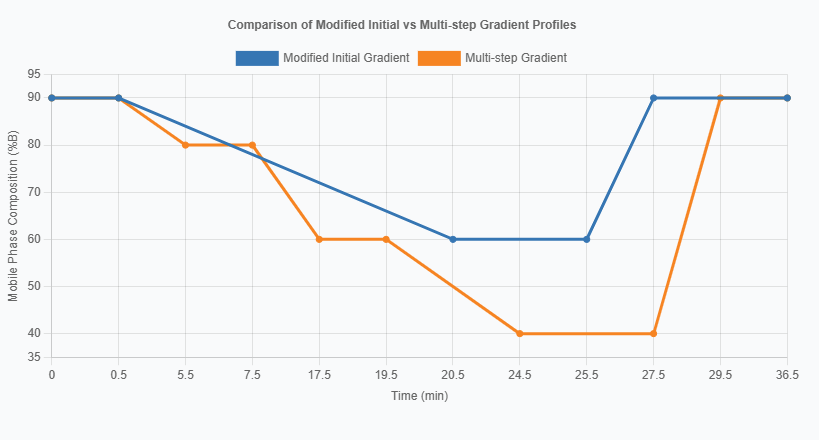
Potential Gradient Conditions for Linear-Gradient Elution
| Time (min) | Mobile Phase %B | Description |
|---|---|---|
| 0.00-0.50 | 90 | Initial Hold |
| 0.50-20.50 | 90 → 60 | Linear Decrease |
| 20.50-25.50 | 60 | Hold |
| 25.50-27.50 | 60 → 90 | Return to Initial |
| 27.50-36.50 | 90 | Re-equilibration |
Potential Gradient Conditions for Step-Gradient Elution
| Time (min) | Mobile Phase %B | Description |
|---|---|---|
| 0.00-0.50 | 90 | Initial Hold |
| 0.50-5.50 | 90 → 80 | Gentle Decrease |
| 5.50-7.50 | 80 | First Hold |
| 7.50-17.50 | 80 → 60 | Linear Decrease |
| 17.50-19.50 | 60 | Second Hold |
| 19.50-24.50 | 60 → 40 | Final Decrease |
| 24.50-27.50 | 40 | Hold at 40% |
| 27.50-29.50 | 40 → 90 | Return to Initial |
| 29.50-36.50 | 90 | Re-equilibration |
¶ Comparison of Buffer Strength and pH Effects on Chromatography Modes
This document summarizes the impact of buffer concentration and pH on three common chromatography modes: ZIC‐philic (HILIC), Normal HILIC, and Reverse Phase. Each mode exhibits different responses to changes in buffer strength and pH based on the nature of the interactions governing retention and separation.
¶ Summary Table
| Chromatography Mode | Buffer Strength | pH | Retention Behavior | Peak Shape / Separation Efficiency | Notes |
|---|---|---|---|---|---|
| ZIC‐philic (HILIC) | Low (e.g., 5 mM) | Acidic (e.g., 3) | Reduced ionic interactions; lower retention for polar analytes due to diminished electrostatic pairing. | Broader peaks may be observed because of less stable pH conditions. | May need to adjust organic modifier strength if retention is too short. |
| Low (e.g., 5 mM) | Neutral (e.g., 7) | Moderate retention; the neutral environment promotes balanced electrostatic interactions. | Good peak shape if buffering capacity properly maintains pH stability; however, limited buffering may lead to variability. | Suitable for analytes that do not undergo extreme ionization. | |
| Low (e.g., 5 mM) | Basic (e.g., 9) | Increased deprotonation of analytes and stationary phase enhances electrostatic interactions, resulting in higher retention. | Tailing peaks may occur if the balance is not optimized. | Watch for ion suppression in downstream MS detection. | |
| High (e.g., 20 mM) | Neutral (e.g., 7) | Enhanced buffering leads to a more stable stationary phase, optimizing separation for a range of analytes. | High efficiency with symmetrical peak shapes as the pH and ionic strength are well controlled. | Often preferred when method robustness is key. | |
| High (e.g., 20 mM) | Basic (e.g., 9) | Further increased retention of deprotonated analytes; can lead to longer retention times as ionic interactions are maximized. | Peak broadening or tailing can be evident if the balance between buffer capacity and analyte charges isn’t optimal. | Optimization is crucial; may need to adjust the organic modifier concentration. | |
| Normal HILIC | Low (e.g., 5 mM) | Acidic (e.g., 3) | Limited interactions between the water-enriched phase on the stationary phase and the organic-rich mobile phase, leading to lower retention. | May exhibit broader peaks due to a less defined water layer; variability in retention times can occur. | Careful fine-tuning of the buffer may be needed to enhance the water layer. |
| High (e.g., 20 mM) | Neutral (e.g., 7) | Stronger buffering stabilizes the water-enriched layer, increasing retention times in line with analyte hydrophilicity. | Yields more reproducible retention and improved peak symmetry due to a well-defined water layer. | Most effective when consistent pH maintenance is required. | |
| High (e.g., 20 mM) | Basic (e.g., 9) | Enhanced ionization leads to increased retention for polar and ionizable compounds as deprotonation of analytes is favored. | Potential for tailing peaks if the balance in the water layer is disturbed, with adjustments needed for optimal separation. | May demand higher organic content adjustments to counter long retention times. | |
| Reverse Phase | Low (e.g., 5 mM) | Acidic (e.g., 3) | Under acidic conditions, basic analytes remain protonated thus resulting in diminished hydrophobic interactions on the nonpolar stationary phase. | Peaks might be less retained and show reduced separation efficiency, with potential loss of resolution. | Buffer concentration here mainly ensures pH consistency rather than modulating hydrophobicity. |
| Low (e.g., 5 mM) | Basic (e.g., 9) | Under basic conditions, increased deprotonation of acidic groups enhances interactions with the stationary phase, leading to higher retention. | Improved separation if the analytes interact properly with the hydrophobic surface; however, results can vary based on analyte structure. | May require modifications to the organic modifier strength to balance retention times and maintain peak integrity. | |
| High (e.g., 20 mM) | Acidic/Neutral | Enhanced pH stability with high buffering capacity, ensuring consistent hydrophobic interactions and reliable retention behaviors across multiple runs. | Provides symmetrical peaks and reproducible retention times, particularly important in robust method development. | Optimal for achieving consistent performance, though excessive ionic strength could interfere with analyte partitioning if not balanced well. |
¶ Detailed Explanation
-
Buffer Strength Effects
- Low Buffer Strength (e.g., 5 mM):
- Provides minimal ionic strength leading to reduced electrostatic interactions.
- In HILIC, this may result in broader peaks and increased variability in retention.
- In reverse phase, the low buffering capacity primarily ensures basic pH maintenance but may not optimize hydrophobic interactions.
- High Buffer Strength (e.g., 20 mM):
- Enhances ionic interactions and stabilizes pH during the run.
- Leads to more reproducible retention times and improved peak shapes across all modes.
- Requires careful optimization to prevent over-strong ionic interactions, which can manifest as peak tailing or excessively long retention times.
- Low Buffer Strength (e.g., 5 mM):
-
pH Effects
- Acidic Conditions (e.g., pH 3):
- Typically suppress ionization of basic groups.
- In ZIC‐philic and HILIC modes, this can result in lower retention for polar compounds.
- In reverse phase, analytes might exhibit reduced hydrophobic interactions, lowering retention.
- Neutral pH (e.g., pH 7):
- Provides a balanced ionization state, stabilizing both retention and peak shape.
- Generally offers the best compromise for reproducible chromatographic performance.
- Basic Conditions (e.g., pH 9):
- Promote deprotonation of analytes, increasing hydrophilicity in HILIC modes and enhancing retention of acidic analytes in reverse phase.
- Can lead to increased retention times and may induce peak tailing if not properly optimized.
- Acidic Conditions (e.g., pH 3):
-
Chromatography Mode Specifics
- ZIC‐philic (HILIC):
- Employs a zwitterionic stationary phase sensitive to both pH and ionic strength.
- Buffer strength and pH changes concurrently alter electrostatic and hydrophilic interactions.
- Normal HILIC:
- Relies on partitioning between an aqueous layer on the stationary phase and an organic-rich mobile phase.
- Buffer conditions are crucial in forming a stable water layer which is central to the retention mechanism.
- Reverse Phase:
- Driven by hydrophobic interactions, where the primary variable is the ionization state of the analytes.
- Proper pH control ensures that analytes display the intended interaction with the nonpolar phase.
- ZIC‐philic (HILIC):
This Markdown document is formatted to be both clear and modern, making it easy to integrate into documentation or presentations.